What are some of the research projects you are currently working on?
I’m finishing up a big project that is looking at an iGEM inspired program that I launched during the pandemic to get students to do team-based learning similar to iGEM, and it was incredibly successful. This is research that I’ve spent most of my time on for the last three years, and I’m continuing to build the program and improve it in various ways. I’m just about to finish a paper, and I’m really excited about it. I can’t continue to research in this space because there’s no funding for it. So that's a little bit sad, but it's the right thing to do.
I also have two human health therapeutics projects in collaboration with scientists at the university. In one study, we want to improve the quality of pluripotent neural stem cells for cell-based therapeutics for neuroregeneration. What we want to do is try to use multiplex CRISPR to activate genes that are normally expressed in neural stem cells, but not expressed in induced pluripotent stem cells. That's a big issue because they look like neurons, but they’re not, so they’re not really good for transplantation. Even though these tools have been around for a long time, they’re not good enough yet for these particular applications. The efficiency is not high enough, and we know too little about it. What we want to do is target five or six transcription factors, which means that each of those guide RNAs has to be very efficient. We are hoping to be able to use yeast as a platform and do some large-scale screens to identify highly efficient guide RNAs. That involves a combination of cloning, bioinformatics, and maybe some artificial intelligence (AI) later on.
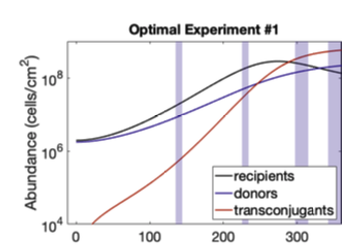
The second project, the long-term goal is to create microbial biosensor systems. These are complicated scenarios, but they are based on an idea where we have two chimeric antigen receptors that each have a binding site for different areas on a protein or virus, and only if those two come together, we will form a complex that then generates a biological signal. Individually, all those components are known, but they haven't been put together. We want to use that for a biosensor because we've used antigen nanobodies, and we can customize it to anything. We’re starting out with spike proteins because we already have so many different antigens available, so we can see if we can get it to work by optimizing that. Hopefully, we will be able to integrate it with intracellular signaling so we can create synthetic signals to junction pathways. One day, we will be able to have cells that have both the guide RNA machinery that can perform complex regulation of gene expressions and have a synthetic signal to junction pathways. That's where we get into my idea of creating smart biological devices which are cells that know where they are, sense their environment, and make decisions based on those signals.
What inspired you to get involved in synthetic biology?
I have a very hard time not understanding what I’m working on. So, what I find really frustrating about certain areas of biological research, is that the knowledge that is generated is very incremental and doesn't necessarily yield an understanding of how a system works. You can identify another phosphorylation site, on another protein, encoded by another gene, and you see some effect, but the ability to understand why you see that effect is extremely limited. The only thing you can really do is record what changes when you are disrupting the system. That’s knowledge, but it's not understanding. Understanding the causal relationship and how the system works is still beyond us, and I find that incredibly unsatisfactory. Synthesis is the highest level of understanding, it's the ability to use knowledge or data or information to create something. And that’s why I like synthetic biology. I thought if I can’t understand it by dissecting it, maybe I can understand it by building it. I first did chemistry in Toronto where I worked with infusion reaction systems where you are not just looking at a chemical reaction in a well-stirred environment, but you’re looking at an environment where you have flows, diffusions, turbulence, and all kinds of fascinating stuff that we know is important to life. But, we're still treating systems as these well-stirred chemical reactors, which is kind of nonsense, but it works remarkably well. Then this opportunity came about with the first programmable cells. So as a postdoc, I worked on creating the first prototype of programmable cells. We actually rewired the cellular machinery to respond in a certain way to an external signal. So that was very exciting. And at the time the fab was systems biology, and that is how I got to Ottawa.
What is BioGroupe Canada?
BioGroupe Canada is a non-profit organization that specifically offers a training program for undergraduates as an alternative to the iGEM competition. Taking some of the key elements of iGEM which is a vehicle for student learning and then addressing some of the deficiencies with the program, not only cost but also the disconnect between institutions and the biotech industry.
What inspired you to start BioGroupe Canada?
We lost funding for iGEM years ago in Ottawa. In 2018 or 2019 was the last time that I helped the team, and the students were really disappointed because they didn’t get to do much. There’s nothing tangible that they gained from it other than some lab experience. So I created another program during the pandemic which replicates many of those things and addresses some of those issues.
We also have to change the way we've been doing academics. One of the really impactful moments I had was when I was at the mall changing my Fido phone, and the person that was helping me said he had an Undergraduate degree in Biomedical Science from the University of Ottawa. He looked at me and said it was the worst decision of his life. So here’s a kid, with so few transferable skills in the real world. Probably with a lot of debt and a lot of regret. In these programs at the University of Ottawa, we are graduating around 1000 students, and it is really hard to see the transferable skills for 80% of them. It was embarrassing to me that we can’t do anything better for our students. That's when I realized we have to change how we do things in our undergraduate training. That's why I created this organization.
I’ve been working with some undergraduate students here in Ottawa because universities don't have resources for this either. It’s kind of like a student club. Hertek Gill has been in charge of developing a number of supplementary learning activities for undergraduates that can help provide and facilitate some of that skills training. The way that we're doing it here in Ottawa they have a student club that we call Bio Connect, and we are hoping to simulate what we had with iGEM where each university has its own cohort of students that would be able to participate in these activities but also propagate them at their own sites. But we're still working on developing these high-quality products, so we don't want to grow too fast because we want to have a strong core that can realistically be distributed.
You need to do something in outreach in terms of getting students involved early on and that requires a lot of work. That was what iGEM was for, but it's not working anymore. I would not advise any students today to launch an iGEM team. It’s just not worth it. The iGEM program is not structured in a way where you could get funding as a work-integrated learning program in Canada. It's not meeting the bar in terms of what is needed for student training. In many cases, synthetic biology stops. Like in fermentation, you create your bacteria, and then synthetic biology ends, and then other things have to take over in order to build the fermentation scale and marketing. You need to be able to offer training that allows students to reach either way. Let us say synthetic biologists have an entrepreneur mindset, which is inherent in synthetic biology, but also have these tools and skills that look for real-world opportunities and look realistically at translating this. Through iGEM you're not connected to a problem that is relevant to the industry in your area. Who is going to teach you the applications and what is needed for translation if you don't have that connection? You can't translate your skills into something that actually matters outside the academic program. And that's why I’m trying to change that.
You initiated the uOttawa iGEM undergraduate training program, what do you think is the key to having a successful iGEM team?
Depends on how you measure success. It’s very difficult to be competitive in iGEM unless you are a state sponsored team that puts money into it because they want to advance on the international stage. There’s simply too much work to do in order to be competitive. The idea of winning the competition is something that successful teams need to put out of their mind quite early. The most successful teams in my opinion are the ones that know why they're there. For individual team members, what is it they want to learn? What are the skills and competencies they want to develop? Also, the ability to work within a team, and find a way to collaborate within a very diverse team to be able to accomplish those learning goals while pursuing what appears to be a common direction. I would say that is how I believe students can get the most satisfaction, because they actually get something tangible out of it. Finding good academic advisors is very key, as maintaining social cohesion, and finding a way where everybody can be seen, heard, and contribute to doing work that is meaningful.
What do you see in the future of synthetic biology?
We have to tap into different resources. What does synthetic biology offer on its own that is unique, that you won't find in any other field? I know we can talk about synthetic biology as being integrated, but it doesn't have anything unique, in my opinion. In order to fund such programs, we have to demonstrate that there's a tangible benefit to those fields. We have to focus on the applied aspect, instead of thinking of synthetic biology as being something that stands on its own. Governments are worried about employment, and companies are worried about revenue. Those are what drives investment in science, research, and development. How can we generate jobs? How can we generate revenue? How can we increase the quality of life? We have to frame it in that way and build up the field based on regional strengths. For example, in Guelph, we would focus on the agricultural side, whereas Sick Kids is already focusing on anti-cancer therapies and oncolytic viruses. That's where I see that it's going. Training institutions will develop incorporating synthetic biology in ways that speak to their local strengths.
Do you think that Canadian research in synthetic biology is keeping up with other nations? What needs to change?
There is a reason why iGEM is in France, there is a reason why Tom Knight gave up his job at MIT to work at Ginkgo, and there is a reason why Drew Endy moved to Stanford. I think that Canada has done quite well. We have a really good return on investment compared to the U.S. It's not as exciting, it’s not as flashy, and it’s slower. We are lacking in the support where young entrepreneurs can go out, and have money to set up a lab and provide for themselves and their families for two to three years, without any expectations on return of investment. The U.S. is doing that, and it costs them billions of dollars, but that is earned home because one in twenty of those companies is gonna succeed and revolutionize. Some of it trickles down to synthetic biology. But that I think is the core issue, that we don't have the ability to create those entrepreneurial environments within academic institutions. Because if you do it this way, then the young entrepreneurs will come back to academia, and academia will be able to understand what skills and competencies are needed to be successful. That is an enormous hole that we have in Canada, in terms of supporting innovation in the bio-economy sector. We are doing well in Canada, and there is a lot of movement on specific initiatives, CAR-T cells are one, but all of those are transaction based, and once you see there's a big market, the government doesn’t care what you call it. You can call it genetic engineering, synthetic biology, or whatever. If it creates jobs, revenue improves the quality of life and helps Canadians, the government is interested. And it's up to us to demonstrate how synthetic biology is able to contribute to that. I think we need to not be so risk averse, provide more funding into entrepreneurial mindsets, and give young entrepreneurs a chance for a few years to build something they're passionate about and have the skills and abilities to do. But we're always looking for matching funding. We’re missing an entire layer of funding for people that innovate outside of the box. We are relying on existing companies wanting to invest in that. That's not innovation.
Dr. Kaern’s research in improving the quality of pluripotent neural stem cells and developing microbial biosensor systems are important advances in the field of synthetic biology. His organization, BioGroupe, will provide students with many opportunities to develop their skills training and gain some valuable experience as they continue their educational career, building a generation of professionals whose skills translate beyond their academic program as they enter the workforce. Dr. Kaern’s work can help the field of synthetic biology integrate into many different fields, accelerate innovation, and grow the economy.





































![Figure 1: BioBits™ Bright is a synthetic biology learning kit that provides exposure to molecular biology and engineering techniques at a beginner level of understanding (Reprinted from [Science Advances] Stark et al 2018).](https://images.squarespace-cdn.com/content/v1/5a00c234f14aa176e49e19f4/1582825656386-ONKZOHNAZK3JQQC9L473/F1.large.jpg)
![Using BioBitsTM Bright, the design, build, and test cycle is implemented to create unique designs using varying levels of fluorescence (Reprinted from [Science Advances] Stark et al 2018).](https://images.squarespace-cdn.com/content/v1/5a00c234f14aa176e49e19f4/1582826269406-FVVFGR76BWN95497YYX5/F4.large.jpg)














